
Nama Senyawa Cbr4 Brain
May 27, 2023 by Jay Rana CBr4 is a covalent (nonpolar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, C is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound.

The carbon tetrabromide molecule, CBr4, is A a polar molecule with
Why is a C-Br bond considered polar? From electronegativity considerations, both carbon and bromine have very similar electronegativities - 2.5 and 2.8 - respectively. Nonetheless, I am told that such a bond would be polar. We generally classify bond between atoms with EN differences < 0.5 as non-polar; why would C-Br be an exception? bond polarity
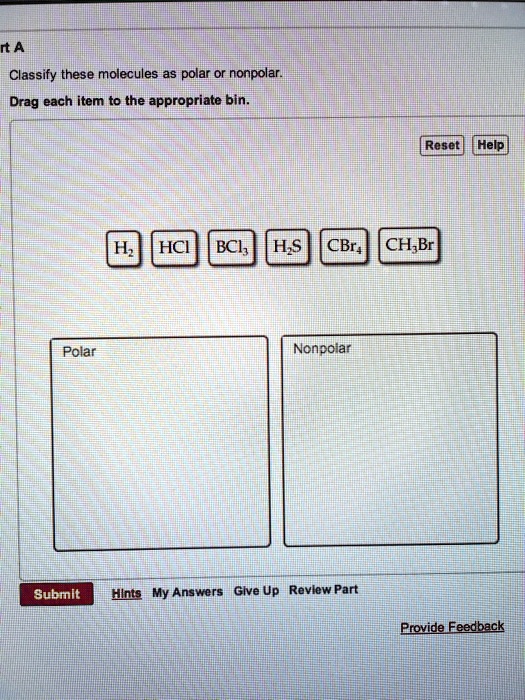
SOLVED rtANClassify these molecules as polar or nonpolar Drag each
Properties of Carbon tetrabromide It has a molar mass of 331.627 g·mol −1. It is insoluble in water. It has a boiling point of 189.7 °C and a melting point of 94.5 °C. It is denser than water. It has a monoclinic tetrahedral structure. Page Contents show How to draw lewis structure for CBr4?

Is CBr4 Polar or Nonpolar? Techiescientist
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.
Cbr4 Electron Dot Structure
The CBr4 molecule is non-polar due to its symmetrical tetrahedral geometry. The four bromine atoms are arranged symmetrically around the central carbon atom, with each bond angle at 109.5 degrees. This results in an even distribution of charge throughout the molecule, with no net dipole moment.
Cbr4 Electron Dot Structure
IUPAC Standard InChIKey:HJUGFYREWKUQJT-UHFFFAOYSA-N. CAS Registry Number: Chemical structure: This structure is also available as a 3d SD file The 3d structure may be viewed using. Other names: Methane, tetrabromo-; Carbon bromide (CBr4); Methane tetrabromide; Tetrabromomethane; CBr4; Carbon bromide; Bromid uhlicity; UN 2516; NSC 6179.
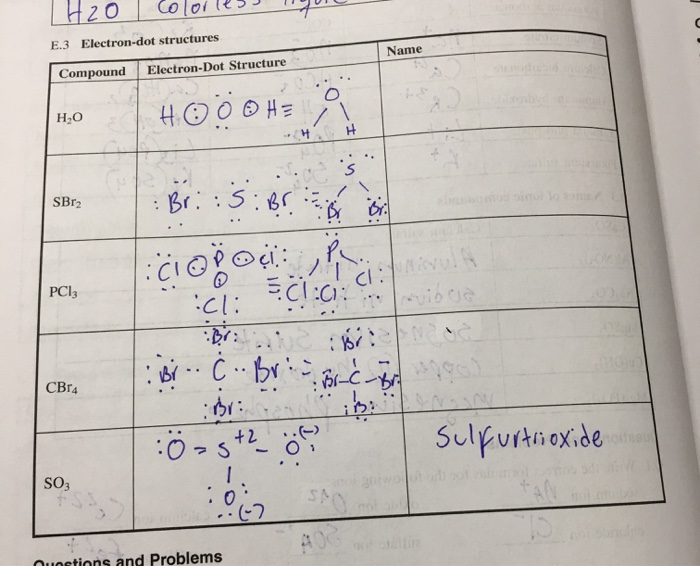
Cbr4 Electron Dot Structure
The molecule CBr4 is non-polar. This is because the charges from the bromine atoms cancel out, resulting a neutral charge. The molecule CBr is called tetrabromomethane, but is commonly known as carbon bromine.
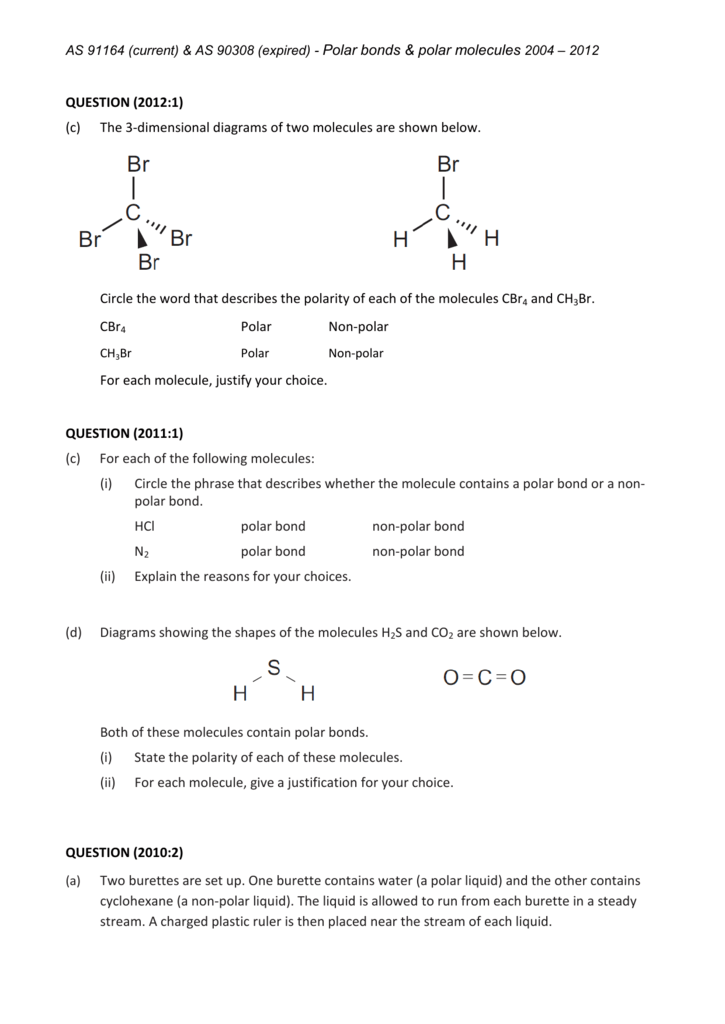
CH Br Polar Nonpolar
The C-O bond is considerably polar. Although C and S have very similar electronegativity values, S is slightly more electronegative than C, and so the C-S bond is just slightly polar. Because oxygen is more electronegative than sulfur, the oxygen end of the molecule is the negative end. Chloromethane, CH 3 Cl, is another example of a polar.

Is CBr4 Polar or Nonpolar? (Carbon Tetrabromide) YouTube
May 24, 2023 by Jay Rana CBr4 is a NONPOLAR molecule. But why? And how can you say that CBr4 is a nonpolar molecule? Want to know the reason? Let's dive into it! CBr4 is a NONPOLAR molecule because all the four bonds (C-Br bonds) are identical and CBr4 has symmetrical geometry which cancels out the bond polarity.

CBR4 Is Best Or Not? SubscribeMe YouTube
The CBr4 molecule is non-polar.. Both CBr4 and CH3Br have four regions of electrons around the central carbon atom. These are all bonding electron regions (clouds) so the shape of both molecules is tetrahedral. The C-Br bond is polar due to the difference in electronegativity between C and Br. Is carbon polar and nonpolar?
Solved 13) Choose the most polar molecule a) CH3Br b) CBr4
Learn to determine if CBr4 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then us.
Ch4 Polar Or Nonpolar Methane Ch4 Polar Or Nonpolar / Is Ch4 Polar Or
Hello Everyone!Do you want to find out if Carbon Tetrabromide is a polar or nonpolar molecule? If yes then check out this video where we share our detailed s.

Cbr4 Electron Dot Structure
CBr4 (Carbon tetrabromide) is nonpolar in nature because of the symmetrical arrangement of four bromine atoms around carbon. As a result, the dipoles of the C-Br bond get canceled by each other resulting in CBr4 a nonpolar molecule.
Cbr4 Lewis Structure
Carbon tetrabromide Carbon tetrabromide, CBr 4, also known as tetrabromomethane, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature . Production CBr 4 can be obtained by the bromination of methane. The byproducts include other brominated methanes ( methyl bromide, dibromomethane and bromoform) and hydrogen bromide.

Ch4 Polar Or Nonpolar / Solution Is The Ch4 A Polar Or Non Polar Chemistry
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.
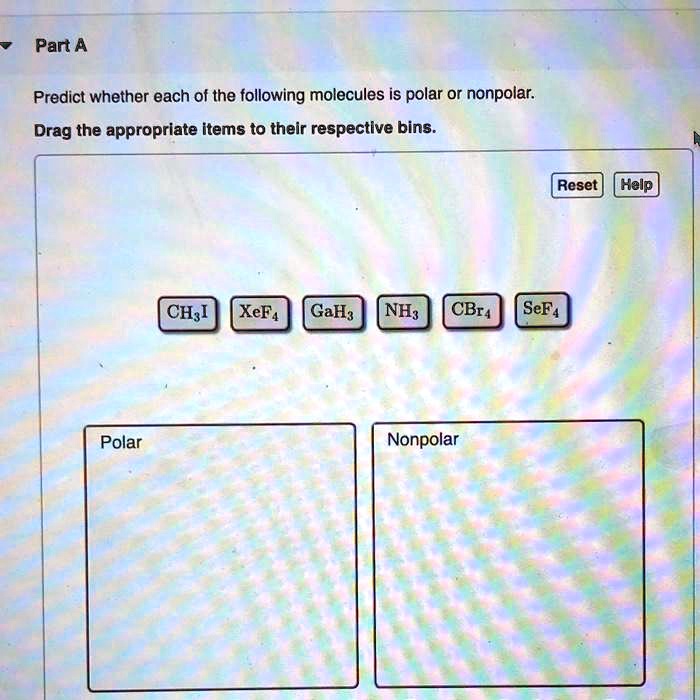
SOLVED Part A Predict whether each of the following molecules is polar
The Lewis structure of CBr4 molecule contains 24 non-bonding electrons that is 12 lone pairs (3 lone pairs are present on each Bromine atom). Details of CBr4 CBr4 lewis structure How to draw Lewis dot structure for CBr4? Following are the steps to follow to draw the Lewis structure of CBr4 molecule